Ko so Jonasa Salka, ki je razvil prvo učinkovito cepivo proti otoški paralizi, med intervjujem na televiziji vprašali, če je vložil zahtevo za patent, se je le začudil in novinarja nazaj vprašal ali je sploh mogoče patentirati kaj takega kot je sonce?
V kratkem dokumentarcu časopisa The New York Times nazorno predstavijo, kako se je farmacevtska industrija spremenila od sredine dvajsetega stoletja do danes.
Dodajamo še nekaj informacij o postopkih razvoja novega zdravila in o stroških kliničnih študij:
Developing a drug normally involves eight stages. At the pre-discovery stage, researchers work on understanding a particular disease. This is followed by what is called drug discovery, in which scientists identify a target or way to combat the disease. They do this by singling out a defective gene or protein, for example, and then they search for a compound that can fight it. They may look at thousands before finding one that is effective. This early work is often carried out by universities or public research organisations …
That phase is followed by pre-clinical testing – mandatory studies in animals to determine the toxicity of the drug. If successful, phase one clinical trials begin in healthy human volunteers to see if the drug is safe. Just over half of all medicines make it to the next round of phase two trials, in which the drug is tested on small numbers of patients to see if it’s effective. Only a third of these make it through to phase three, the next stage of clinical trials, carried out on 1,000 or more patients to provide evidence of effectiveness. About 70% get through. … the final clinical trials are particularly expensive because of the numbers involved, and the cost of manufacture: “The need to produce high quality pure material in large quantities adds enormously to the cost of development.”
Information about the drug then has to be submitted to the regulatory authorities for licensing approval and, at the final stage, the medicine is made available to patients. Just one in 5,000 drug candidates make it all the way from the drug discovery phase to licensing approval. It’s easy to see why it’s expensive – and the new wave of biological drugs are even more costly. (The price of health: the cost of developing new medicines | Healthcare Professionals Network | The Guardian)
Povprečni stroki kliničnih študij na pacienta (vir):
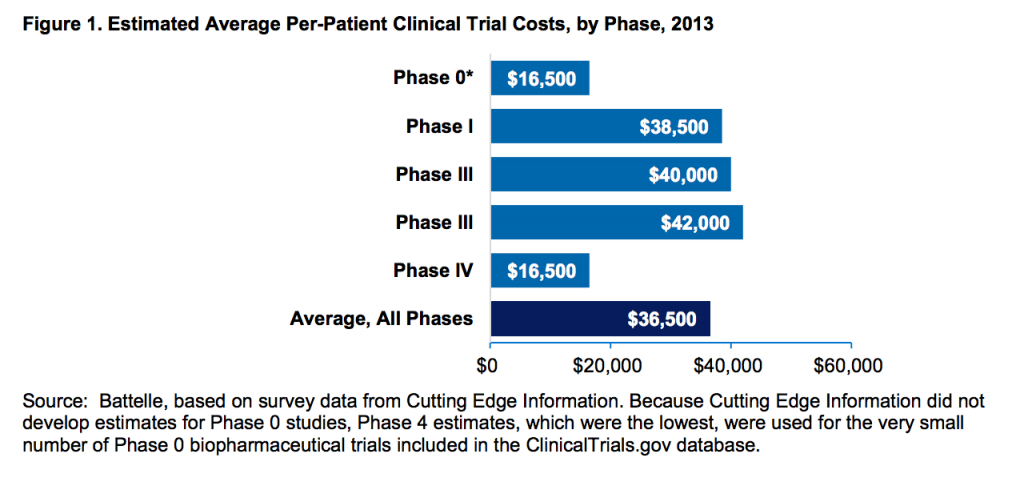
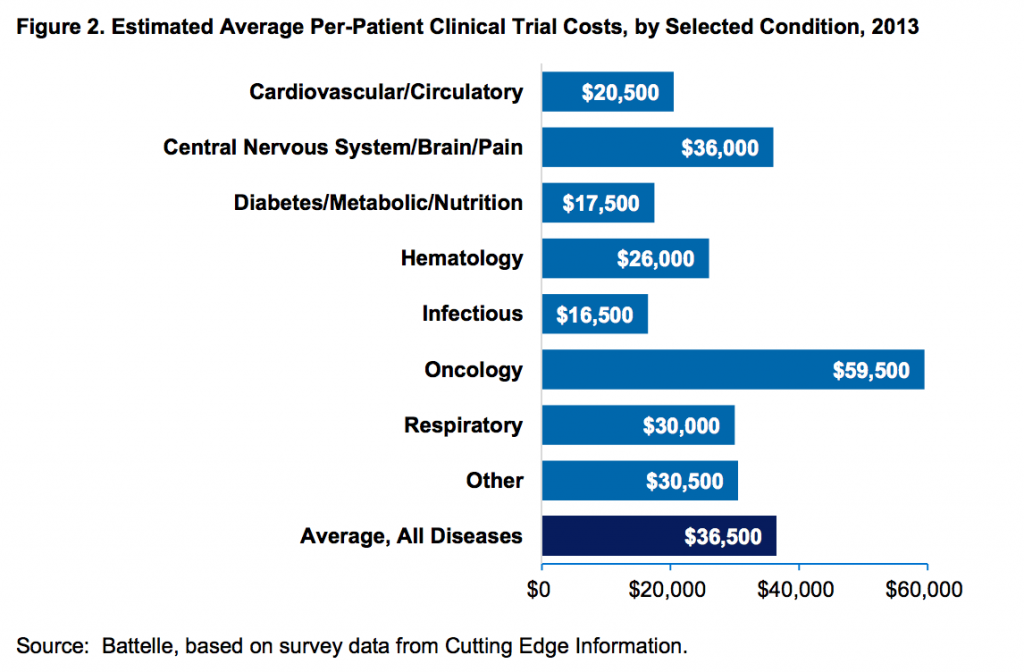
Raziskava o stroških razvoja zdravil, kjer so všteti tudi stroški za tista zdravila, ki na koncu ne pridejo na trg:
The research and development costs of 106 randomly selected new drugs were obtained from a survey of 10 pharmaceutical firms. These data were used to estimate the average pre-tax cost of new drug and biologics development. The costs of compounds abandoned during testing were linked to the costs of compounds that obtained marketing approval. The estimated average out-of-pocket cost per approved new compound is $1395 million (2013 dollars). Capitalizing out-of-pocket costs to the point of marketing approval at a real discount rate of 10.5% yields a total pre-approval cost estimate of $2558 million (2013 dollars). When compared to the results of the previous study in this series, total capitalized costs were shown to have increased at an annual rate of 8.5% above general price inflation. Adding an estimate of post-approval R&D costs increases the cost estimate to $2870 million (2013 dollars). (Innovation in the pharmaceutical industry: New estimates of R&D costs)